Microchimerism: How Your Baby's Cells Can Stay with You for Life
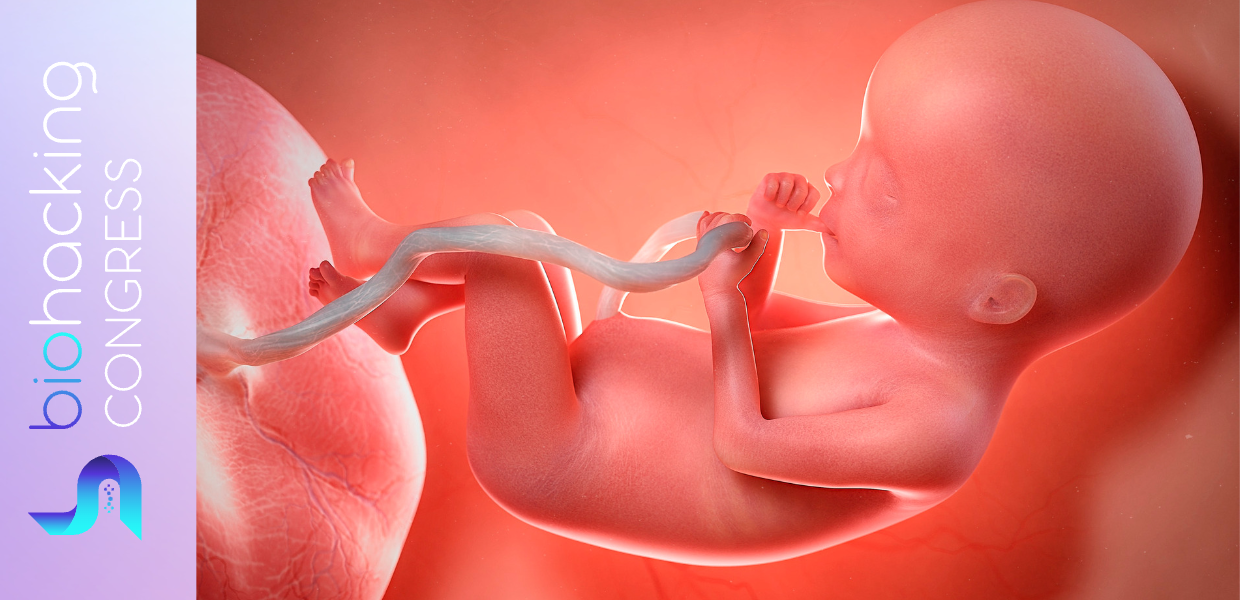
Mothers often describe the feeling that their children are a part of them long after giving birth. This feeling is not just metaphorical - during pregnancy, cells from the fetus cross the placenta and enter the mother's body, where they can become part of her tissues. This cellular invasion means that mothers carry unique genetic material from their children's bodies, creating what biologists call a microchimera, named after the legendary beasts made of different animals. The phenomenon is widespread among mammals, and scientists have proposed a number of theories for how it affects the mother, from better wound healing to higher risk of cancer.
However, a team of biologists argues that to really understand what microchimerism does to moms, we need to figure out why it evolved in the first place.
Maternal-fetal conflict has its origins with the very first placental mammals millions of years ago. Over evolutionary time, the fetus has evolved to manipulate the mother's physiology and increase the transfer of resources like nutrition and heat to the developing child. The mother's body in turn has evolved countermeasures to prevent excessive resource flow.
Things get even more intriguing when fetal cells cross the placenta and enter the mother's bloodstream. Like stem cells, fetal cells are pluripotent, which means they can grow into many kinds of tissue. Once in the mother's blood, these cells circulate in the body and lodge themselves in tissue. They then use chemical cues from neighboring cells to grow into the same stuff as the surrounding tissue.
Although the mother's immune system typically removes unchanged fetal cells from the blood after pregnancy, the ones that have already integrated with maternal tissues escape detection and can remain in mom's body.
Microchimerism can get especially complex when a mother has multiple pregnancies. The mother's body accumulates cells from each baby - and potentially functions as a reservoir, transferring cells from the older sibling into the younger one and forming more elaborate microchimeras. The presence of fetal cells in the mother's body could even regulate how soon she can get pregnant again.

Given all this complexity, microchimeras have been difficult to study until recently. The phenomenon was discovered several decades ago when male DNA was detected in the bloodstream of a woman. But the technologies of the time couldn't get a detailed enough picture of the genetics to tease apart the minute cellular situation.
Now, deep-sequencing technologies allow researchers to identify the origin of DNA in a mother's tissues more comprehensively by sampling many areas in the genome, including genes implicated in immunity. These genes are unique to an individual, and thus can help differentiate a mother's DNA from that of her children with greater precision.
Still, understanding how the fetal cells are interacting with maternal cells is going to be difficult. Little is understood about the cellular signaling that causes fetal cells to regulate maternal physiology.
On the other hand, microchimerism has been linked to potential health benefits for mothers as well. Studies have shown that the presence of fetal cells in the mother's body can improve wound healing and may even offer some protection against certain cancers.
The complex interplay between maternal and fetal cells also highlights the interconnectedness of life and the evolutionary history of mammals.

As research into microchimerism continues, it could lead to new insights into the mechanisms behind maternal-fetal communication and potential health implications for both mothers and children. Ultimately, it may even shed light on the evolution of life itself, as scientists delve deeper into the mysteries of microchimerism and its role in shaping our bodies and our world.
Join BiohackingCongress Miami on October 20-22!
Additionally, if you're interested in learning more about optimizing your body's potential and maintaining good health, we invite you to attend the BiohackingCongress in Miami on October 20-22! Limited early bird tickets are available, so don't wait to reserve your spot. Click the button below to purchase your tickets now with special discount for Mother Day.
Use Promo-code MOM and get 80% OFF.
Get Your Tickets:
Join us for an exceptional experience at the BiohackingCongress Miami, featuring a panel of leading biohacking and longevity experts who will showcase the latest research and insights on how to optimize your life.
Get ready to be inspired by our remarkable speakers and seize the chance to connect with like-minded individuals. Don't miss out on this incredible opportunity to take your personal growth to the next level!
Special Access to
Exclusive TopBiohacks
and more


