Deuterium Depletion, the greatest discovery in biology of our time
“We're going to start at the very beginning, which is the beginning of the universe. At the beginning of the universe when matter started forming the big bang you had the first element. The second element to form was helium. The difference is that between the hydrogen there was something called deuterium. Now deuterium is an isotope of hydrogen, the difference is that regular hydrogen has one proton and deuterium has a proton and a neutron. This is very significant because when you have something that has a neutron where something doesn't in this particular case it means that it's twice the mass. So deuterium is a form of hydrogen that has twice the mass of regular. The reason deuterium was formed is a trend of the first transition element because you need that neutron to create the second element which is helium. Deuterium as the universe cooled got stuck so we have this isotope of hydrogen that was never supposed to be here, but it got stuck because the universe cooled too quickly and still we have the same amount of deuterium on the planet now or on the in the universe now that we had in the very beginning of time.”
“We live in a water world and the biggest problem with deuterium is it also binds with hydrogen to produce water. In this case, a regular H2O as we know it is two hydrogens and one oxygens and HDO which is hydrogen-deuterium oxygen and so this hdo is the primary problem that we have on our planet. In our biology heavy water D2O is something we make in a lab and doesn't really occur too often.
However, hdo is roughly about a hundred and our drinking water is about 150 parts per million so for every glass of water we drink roughly three drops of that is HDO and not H2O.”
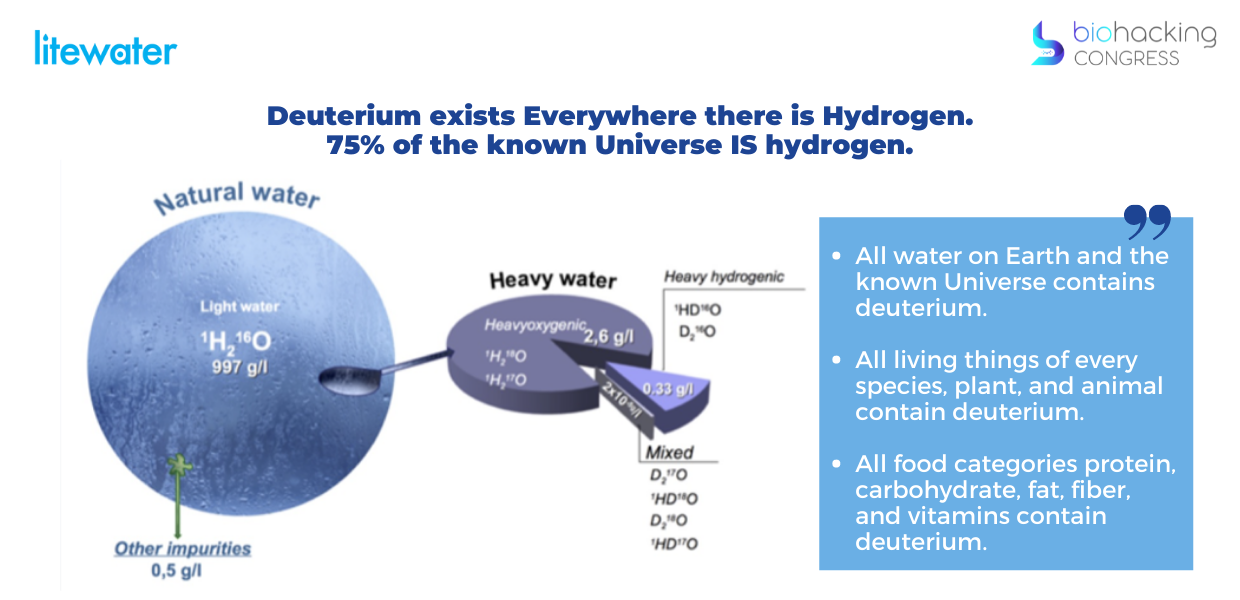
Deuterium exists Everywhere there is Hydrogen. Deuterium was formed soon after the Big Bang when a fraction of hydrogen atoms took up a neutron. 75% of the known Universe IS hydrogen.
- All water on Earth and the known Universe contains deuterium.
- All living things of every species, plant, and animal contain deuterium.
- All food categories protein, carbohydrate, fat, fiber, and vitamins contain deuterium.
“Mitochondria is the powerhouse of the cell. So daily we recycle 1500 gallons of water if you can believe it. All our cellular processes move a lot of water. Because we are producing energy at the same time and our 35 trillion cells have mitochondria. Everything in nature is hungry because the mitochondria needs food and this is because a deal was made 1.45 billion years ago between two primitive cells the eukaryote and the mitochondrion. The deal was that mitochondria creates water, creates ATP energy and the nucleus of the cell protects it as the manager but it also provides the food. So inside the mitochondria, a very complex system that makes this energy and where it ultimately leads to is something called the electron transport chain.”

“The electron transport chain has at the end of it basically shuffles protons and electrons to make ATP adenosine triphosphate which is the energy currency of our biology and at the end of this electron transport chain you have something called ATP synthase nanomotors.”
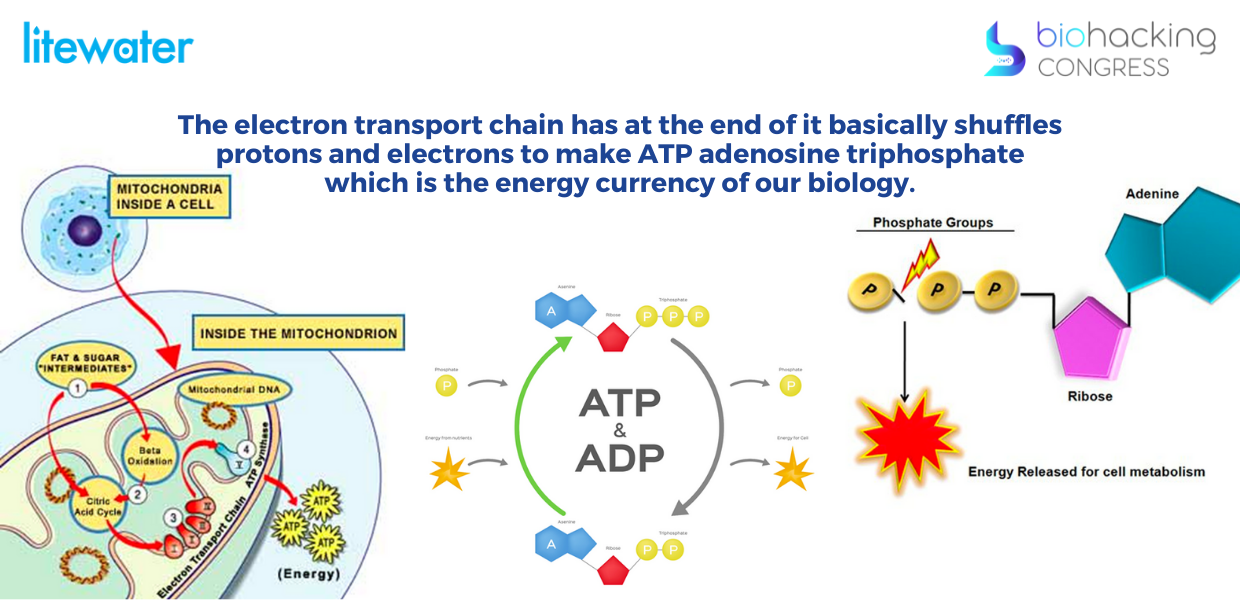
“Each mitochondria has more than 300,000 ATP Synthase nanomotors. ATP Synthase - the smallest nano machine in nature. ATP Synthase spins at a limit 9000 RPM, at perfect 100% efficiency, shuttling 60,000 protons per minute to make ATP and metabolic water. Our mitochondria and these specific nanomotors are specifically damaged by the deuterium. So once the deuterium enters the cell, the Krebs cycle tries to keep it out; other metabolic processes try to keep the deuterium out of this electron transport chain that ultimately goes to these motors. But it cannot. So eventually you get overwhelmed with too much deuterium. Once the deuterium in water enters the cell and becomes accessible to energy-generating mitochondria, it functions as an isotopic toxin that is ultimately fatal to the inner mechanisms of the electron transport chain.”
Deuterium composition of water on Earth
- 2.5 to 2.75% of Earth's water is freshwater.
- 75% of this is frozen in glaciers and icebergs.
- Only 1% is available for all living things and their needs.
- 99.9% of humans consume water with 145 - 155 ppm deuterium.

Where was this discovered, how do we know about this?
“It began in the 1950's, about the same time Watson and Crick announced the double-helix structure of DNA. A gerontologist and biophysicist at the Medical Institute in Tomsk University, Siberia, began to investigate a very peculiar anomaly concerning the lifespans of the Russian population. While the average percentage of centenarians (living over 100 years) in all of Russia was about 50 per one million, in mountainous areas of Siberia there were an astounding 324 centenarians per one million, over 6x more than the national average.
Knowing that these long-living people inhabit high mountainous areas where they consume pristine glacial meltwater, he focused on the possibility that some unique water characteristic might be involved.
By 1960 Berdishev & Rodimov had enough information to conclusively link the longevity of the Yakuts and the Altaians with the consumption of glacial meltwater.
It was an especially groundbreaking discovery, considering that deuterium had only been discovered 30 years before and little biological research had been conducted on it.”
Concentration of Key Constituents in Human Body Fluids. There is 3-5X more deuterium in the body than some of the most important constituents needed.”
- Calcium in blood serum 2.24 - 2.74 mmol/I
- Magnesium - 0.75 - 1.2 mmol/I
- Potassium - 3.5 - 5.1mmol/I
- Glucose - 3.3-5.1 mmol/I
- Deuterium - 12 -14 mmol/I
- The consequences of deuterium are intensified because 98.8% of the molecules that make up the body are water!
- 110 lb. a human containing 70 lbs. of body water contains 1.1 grams of deuterium.
- 24 hours a day, our cell's mitochondria are subjected to as many as 458 sextillions potentially damaging deuterium atoms.
How Does Deuterium Damage Mitochondria?
“The transport of 2x heavier deuterium atoms damages the ATP synthase nano-motor, causing a stutter and decreasing cellular ATP production. 10-60 million ATPs are produced per cell with a rate of 1500-10k deuterium disruptions on the ATP Synthase nano-motor per second.”
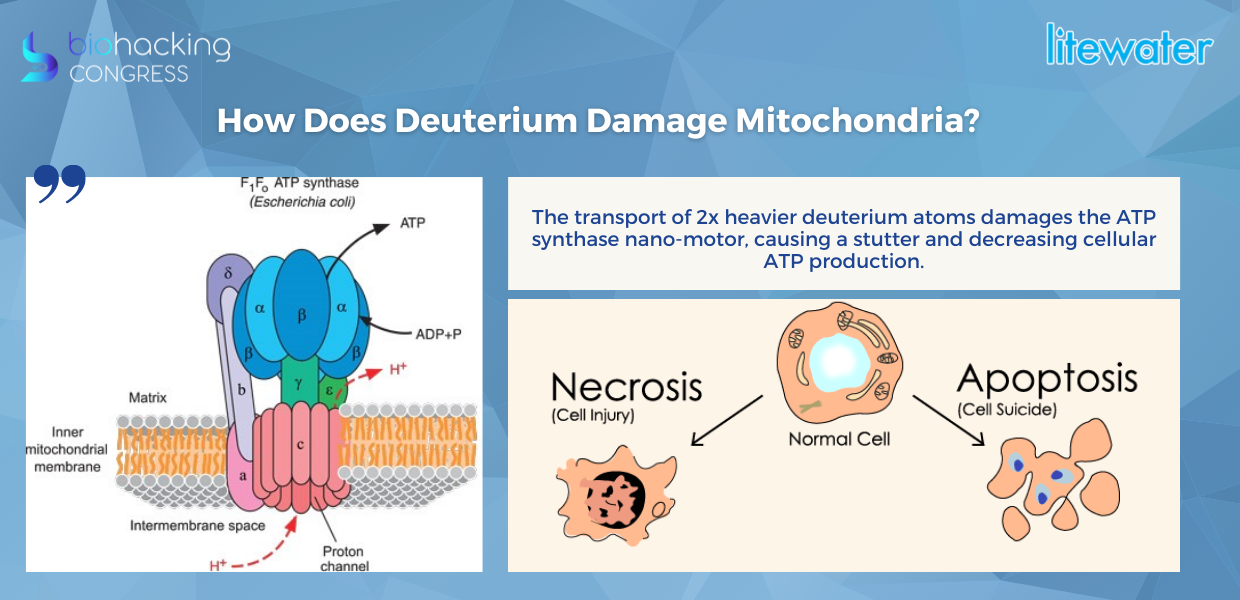
“The reason the mitochondria dies is that the membrane gets compromised not only the permeability of the membrane for nutrient and waste to go out, but also the leakage of the protons and as this happens these ATP motors stop producing and pretty much that's the end. So cells die in two ways: necrosis which there's nothing we can't affect that you get injured cell dies. But what we can affect is apoptosis cell suicide because that happens directly because of this mitochondrial problem, as soon as the mitochondria stop functioning can no longer produce the energy it sends a signal to the cell and when you don't have enough mitochondria in the cell no longer has bioelectricity and therefore it starts to turn off or die."
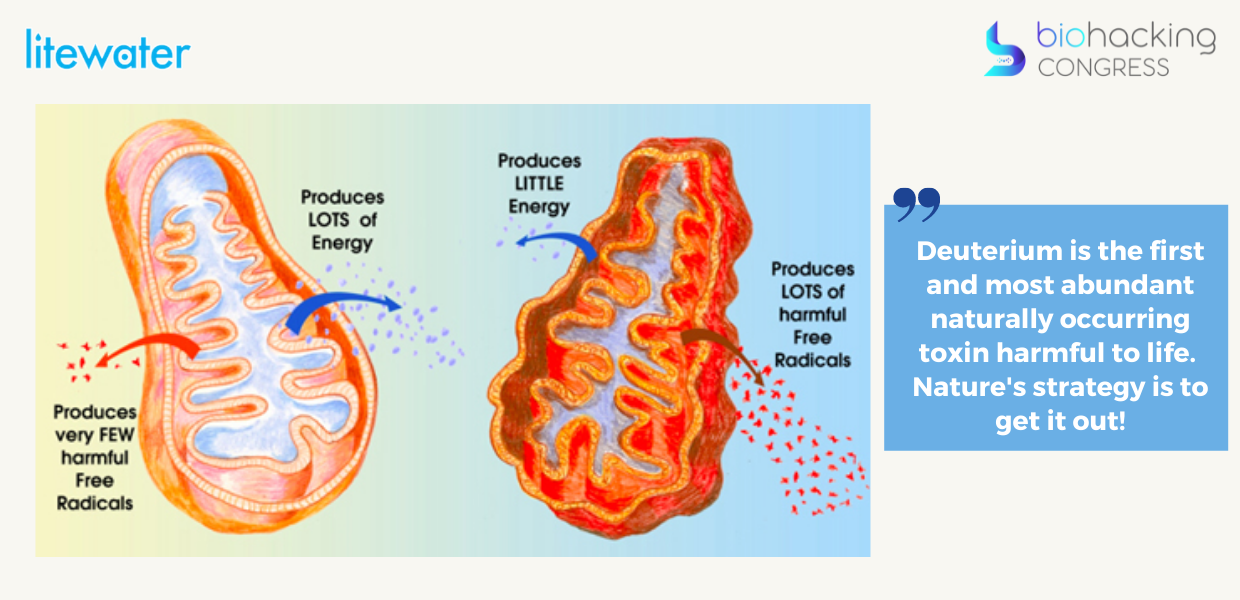
“Deuterium is the first and most abundant naturally occurring toxin harmful to life. Nature's strategy is to get it out! You can see the wolf and sheep's clothes being disguised as that HOD or HDO molecule that looks like water, tastes like water but is not water. The bond between deuterium and oxygen is much stronger. The bond with deuterium can be explained through something known as quantum tunneling.
When you look at where mitochondria is expressed the most, what is the crowning magnum opus achievement of mitochondria - it's the eye. Most mitochondria is found in the eye in the retina so the lens onto which our reality is projected is the place where you find the most mitochondria in nature. So one of the best ways to determine the state of health of your mitochondria is reaction time. If you shine light in your eyeball the quicker it closes the healthier mitochondria.”
Protium Fuels Mitochondria
- Deuterium damages the ATP synthase nanomotors that produce ATP
- Deuterium affects the Electron Transport Chain compromising oxygen carrying capacity.
- Deuterium mechanically distorts DNA and enzyme molecules causing errors in transcription.
- Reduced bio-energetics promotes an accumulation of DNA errors, cellular mutation, and aging.
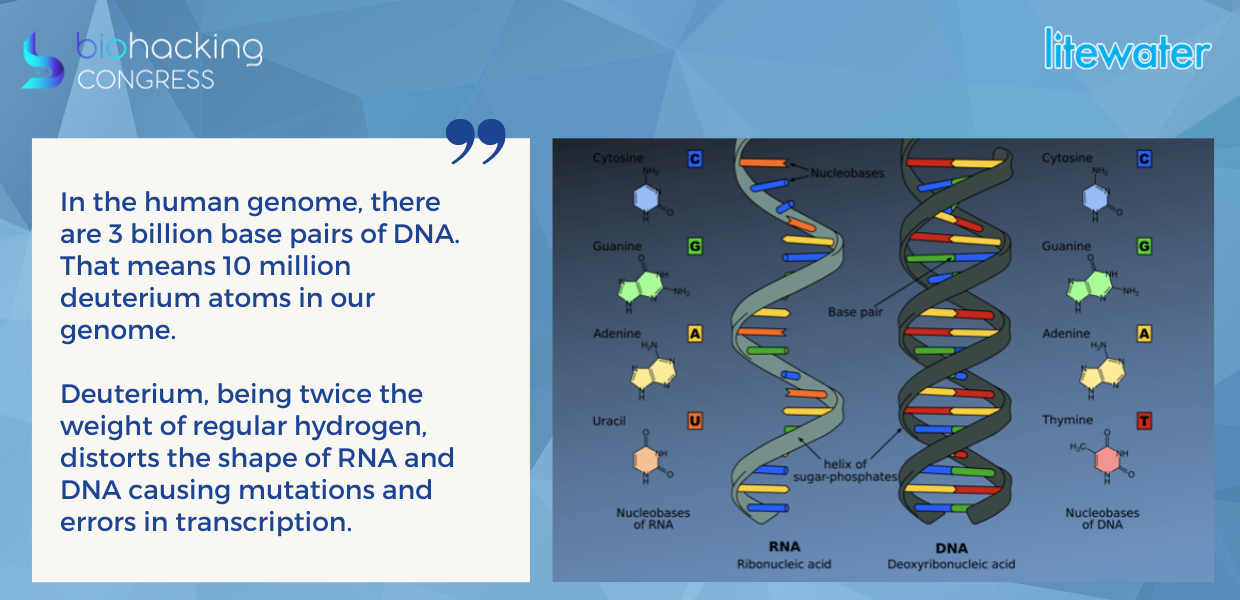
“In the human genome, there are 3 billion base pairs of DNA. That means 10 million deuterium atoms in our genome or 1 deuterium atom per 300 base pairs. Deuterium, being twice the weight of regular hydrogen, distorts the shape of RNA and DNA causing mutations and errors in transcription.”
“You can see as the DNA errors accumulate you have more chances of error in your translation, biohack your health, biohack your longevity, of your stem cells to your normal cells, and then you have more ability to have neoplastic conditions or cancer in your body because you have more DNA errors. Great marker of aging is skin which is skin made up of collagen. So here you have collagen just like the DNA, RNA, enzymes proteins and collagen is coiled up like a spiral.”
“Deuterium adversely affects the shape of the enzyme molecules that are involved in DNA replication. The Possible Roles of Deuterium in the Initiation and Propagation of Aging and Other Biological Mechanisms and Processes, - Griffiths 1974”
“Deuterium in water, food, and the environment has not been constant. Meteorological changes over the past 10,000 years have increased the global distribution of deuterium in liquid water and vegetation. This likely resulted in a corresponding increase in endogenous deuterium in animal species - predicted as much as 30%.”
Deuterium Depletion May Be the Most Important Health and Medical Discovery of our Time.
A Keto Diet increases ATP production and decreases deuterium.
- Glycolysis (w/o oxygen) creates: 2-4 ATP
- 1 glucose molecule creates: 34 ATP
- 1 ketone body creates: 104 ATP
- For every 2.4 lbs. of fat burned 1 liter of DDW is created.
BiohackingCongress Team is very grateful to Victor Sagalovsky, Co-Founder and CEO, Litewater Scientific, for joining the BiohackingCongress and giving a life-changing lecture.
Watch Victor Sagalovsky’s 40-minute lecture and 100+ lectures, panel discussions, and performances from renowned biohackers on biohacking your body with a BiohackingCongress year-long subscription. Get your access to a healthy future now!
Subscribe to watch more Videos with Top Biohackers. Stay tuned into the latest biohacking products for biohacking your health and biohacking for longevity.
Based on the lecture by Victor Sagalovsky
Special Access to
Exclusive TopBiohacks
and more



