Deuterium Depletion: The most powerful biohack you never heard of
“There's a lot of things out there but I think this is the one that really takes the crown, when it comes to people saying ‘anti-aging’ I like to call it ‘pro-eating’. What we need to do is to intercept aging at its root and this is the promise of deuterium depletion.”
“Deuterium is an isotope or a version of hydrogen. Hydrogen is the first element in the universe, it's the simplest element and it still makes up 75 percent of the universe. Everything runs on hydrogen from our bodies to the stars. The difference between protium and deuterium is that deuterium has an extra neutron, as you can see there, and herein lies the problem that causes the hydrogen to be twice the mass of what normal hydrogen is. Deuterium is important so on earth you see how little deuterium there really is. It's very small amount, but when it gets into our bodies you will see the damage that it does”
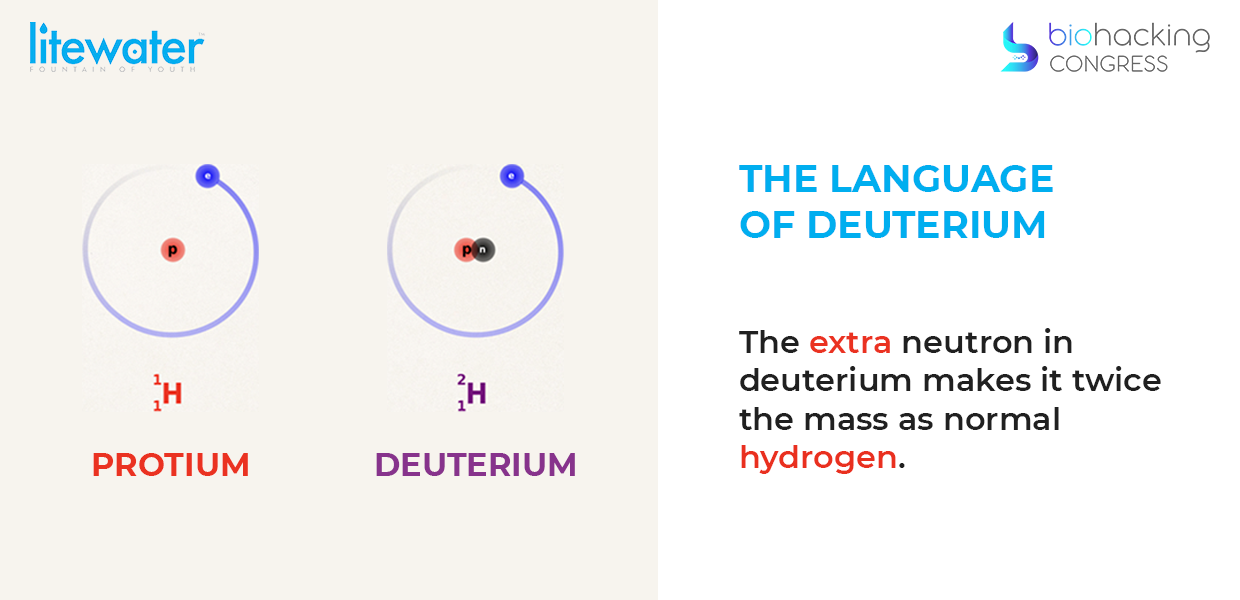
“Water, the most essential and revered nutrient for life, also conceals a little recognized harmful toxin, known as deuterium, an isotope of hydrogen. It's a natural contaminant. Even the most knowledgeable health medical professional is just discovering this serious, but little recognized health risk, it's been overlooked. Unfortunately, it's been overlooked in our biology and so my job in this field of science called 'deuteronomics' is to make people understand how important this phenomenon is. The problem is that deuterium damages the mitochondrial atp mechanisms and I'm going to explain why.”
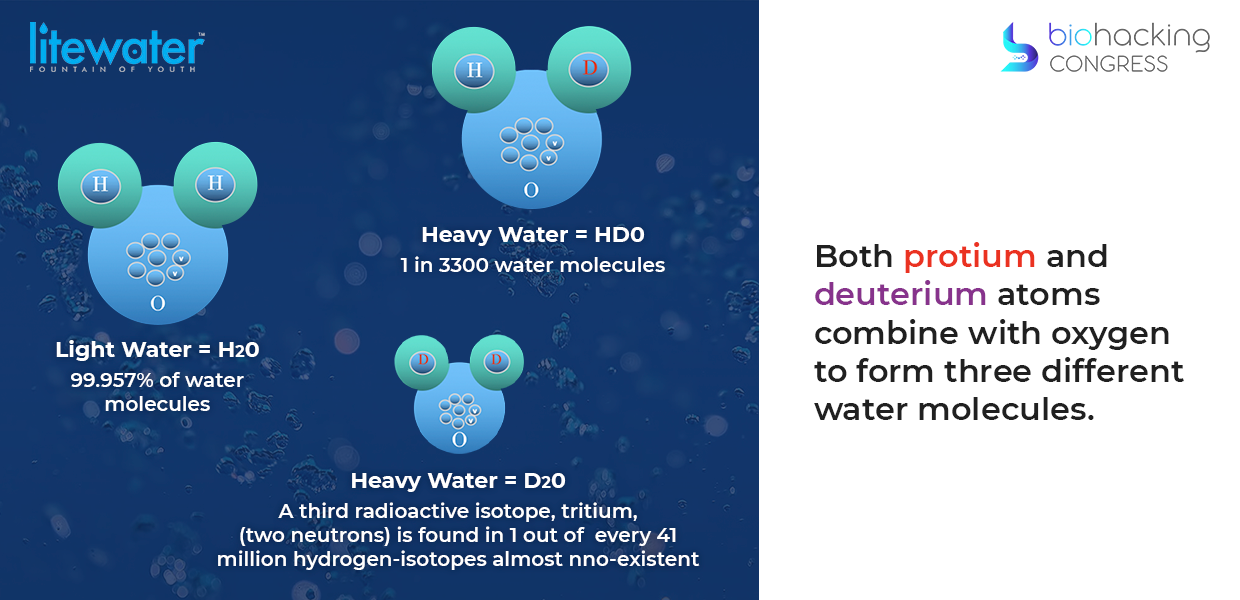
“As I mentioned, this is a slightly more in-depth view of what's going on. Deuterium exists everywhere, there is hydrogen. Deuterium was formed after the big bang so it's been with us since the beginning of the universe and all water on earth contains deuterium. Deuterium is everywhere, essentially it's about in the oceans, for one million parts of regular water, which is H2O you have 150 parts of deuterium.
The problem of deuterium is that like all hydrogen it bonds with oxygen to create water and this is the problem. As you can see in every liter of H2O there's six drops of HDO, so once in a while you get what's called D2O, or heavy water, which is very interesting because without it we wouldn't have the nuclear age.”
“The history of the research began in the 1950s in Siberia. They were trying to figure out why populations that were living in the very harsh environments it's Siberia and yet these people were living six/seven times more centenarians than everywhere else in the Soviet Republics. And after a couple years of study they figured out that: it must be something with the glacial water and then they tested water to prove it and it was 16 to 20 percent lower in deuterium than everybody else's water. In 1961 they published their findings um and this i mean to me this was revolutionary”
“I do a lot of research around this subject and the subject of longevity. Census from the mountains, where they have a deuterium level 15 to 20 percent lower, they have people reaching the ages of 160 years - 1 789 centenarians. When you remove deuterium you get longer life. Now let me back up and say if you have two glasses of water and one is heavy water or D2O and one is regular water you will not taste the difference, you will not notice the difference, but if somebody gives you this for five days you will die, because it will stop your metabolism your mitochondria will shut down and you won't know why because it looks and tastes like water.
“Heavy water is an inhibitor, a decelerator, a reducer of biochemical reactions. It slows things down, the removal of it or the reduction of it speeds things up. It's very simple”
“We're standing on the shoulders of giants here because this is so new. Only in 1931 was it discovered deuterium. In 1942 first use of heavy water and nuclear reaction you're just starting to understand this stuff. Then in 1958-61 in Siberia they're doing experiments with natural deuterium depleted water and they're finding things to grow bigger, grow faster, live longer. Then in 1971 in the late 60s the Americans got Russian studies because the Russians were allowed to publish in English in 1966. In 1971 the first US based research paper on deuterium depletion was covered in nature and then, it wasn't that long ago, that you had the first conference on isotopic and molecular processes and then a pivotal book in 2001 published by Gábor Somlyai called the “Biological effects of deuterium depletion”.”
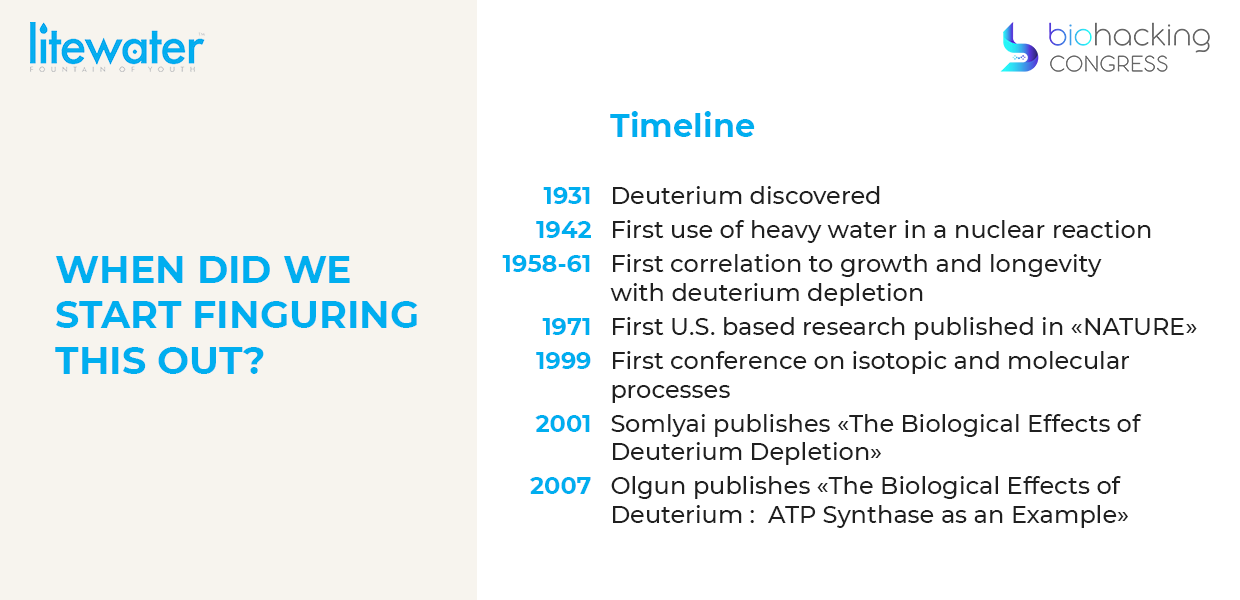
“Then he wrote a book called "Defeating cancer" which is about using deuterium depletion as a strategy as an adjuvant. You increase your mitochondrial health, you increase the energy and that increases your survivability. This was in 2001. It's 2021 he's up to 3 300 case studies now specifically in neoplastic diseases and the conclusion is that it has an 8x survivability factor meaning people that were deuterium depleted survived on average 8 times longer than people that were not this huge. 2007 is a pivotal year, because that's when it was discovered actually how deuterium damages mitochondria, before it was just observed you give something light water it grows bigger faster you give somebody heavier water goes slower, but why what's going on. But so in 2007 Abdullah Olgun professor, doctor of a Medical College in Turkey asked this question he wanted to know if deuterium was harmful to the body. He published “Biological effects of deuteronation: ATP synthase as an example”
“In each cell we have 1000 to 100 000 mitochondria. Mitochondria are little engines, little factories, actually factories that are floating in our cells and those factories came in here 1.45 billion years ago. They're actually from somewhere else they came in and infected eukaryotic, because 1.5 million years ago we were just symbolising simple celled organisms with a dream, but the mitochondria is that's why we have two types of DNA: we have a mitochondrial DNA and we have our nuclear DNA. The mitochondria is like a microbial invader, but it made a deal with all life on this planet: ‘let me hang out on your cells, I'll make you water and ATP and energy’. So the deal was struck and that's why we all have trillions of mitochondria in our body and these mitochondria produce the energy that we need for life”
Timeline
- 1.45 billion years ago mitochondria (a bacteria) invade eukaryotic cells and are genetically entwined with life on Earth forever
- 1890 mitochondria discovered, first called “bioblasts”
- 1898 Term “mitochondria” is coined meaning “thread granul” in Greek
- 1929 ATP discovered
- 1937 Krebs Cycle discovered
- 1960’s Electron Transport Chain and ATP Synthase discovered
“This is the inside of your mitochondria and these are the ATP synthase nanomotors. This nanomotor sits at the end of the electron transport chain which is basically the process by which you create ATP. Here's the interesting thing: it spins at 9000 rpm at 100 efficiency . There is no motor and yet these are the motors that drive our life. They shuttle 60 000 protons per minute to make ATP.
ATP synthase is a molecular machine that works like a turbine to convert the energy stored in a proton gradient into chemical energy stored in the bond energy of ATP. ATP synthase nanomotors are really spinning at 9000 rpm so what happens is these: protons right every all food that we eat, everything breaks down into hydrogen and protons, the protein and these protons come into the channel there's a ph gradient from ins from the mitochondria and the outer membrane space. So that differential causes these protons to flow in and as they flow in they spin this motor, and this motor creates ATP and water and that metabolic water is actually deuterium depleted.”
“Imagine if you wake up in the morning and you kiss your girlfriend/ boyfriend/ wife/ husband/ whoever and they kiss you back, but every once in a while instead of a kiss you get an elbow to the face and a knee to the groin, and that's what deuterium is doing to us from the moment we're born to the moment we're dead.”
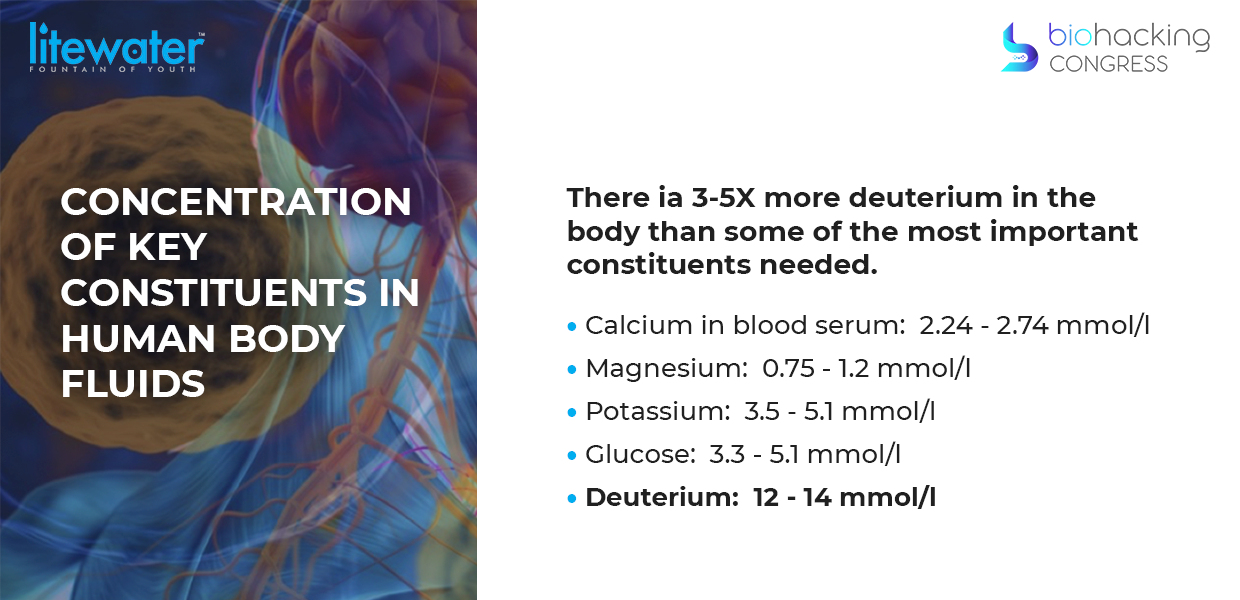
“The first question is why would you even want to look at this problem? Why is it so important? Because everybody else says "it's just six drops in a liter of water. It's no big deal". And it turns out it has like four times more concentration of deuterium in our blood plasma than the very things we need for survival: glucose potassium magnesium calcium etc. So it's even though it's six drops in a liter of water there's a whole lot of it in our body”
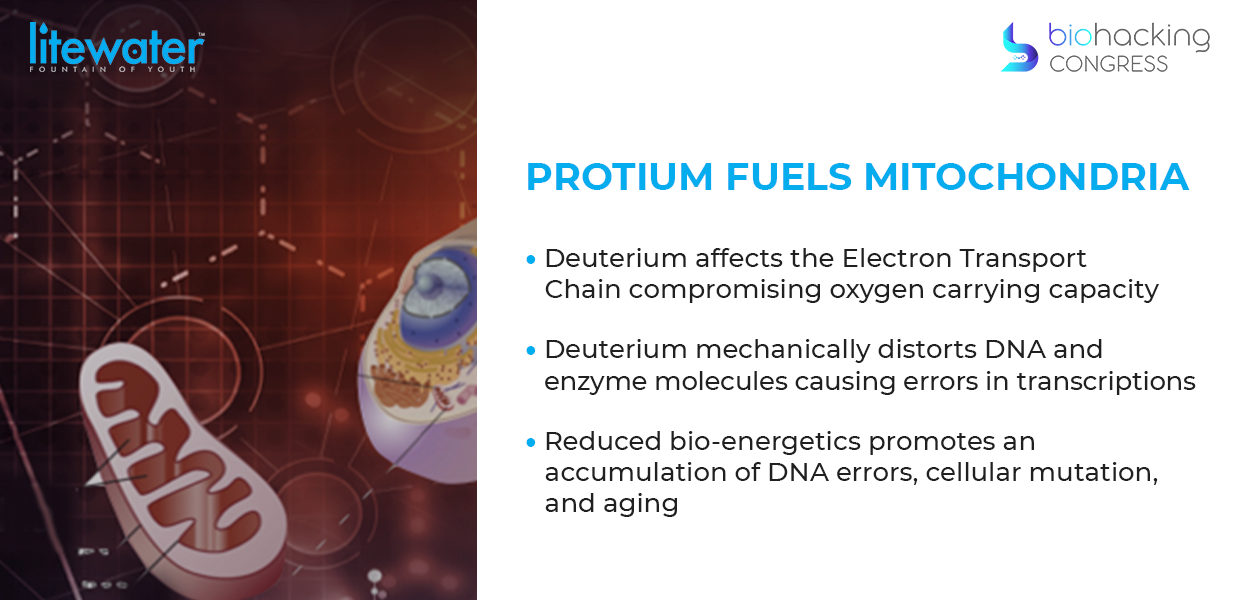
How does deuterium damage mitochondria?
“The transport of 2x heavier deuterium atoms damages the ATP synthase nanomotors, causing a stutter and decreasing cellular ATP production.
10-60 ATPs produced per cell with a rate of 1500-10k deuterium disruptions in the ATP synthase nanomotor per second. It gets you when you're sleeping, it gets you when you're awake.
We know that we're 75 % water but in fact we're 98.8% water by molecular weight, so we are water beings it's no doubt so that's why we have so much deuterium in our body.”
“So during a 24-hour cycle our cells' mitochondria are subjected to as many 458 sextillion potentially damaging deuterium atoms. That's a lot of zeros so it's what I'm just trying to reinforce - it's constant, it's a constant bombardment on our mitochondria and nothing slows it down . Somebody might ask the question: "Well wouldn't have evolved to manage this problem", and in fact we have the cycle of deuterium exchange. It just trades out hydrogen and tries to keep deuterium out of that electron transport chain. However it's cumulative, so over time it just can't keep up.”

Deuterium is the first and most abundant naturally occurring toxic harmful to life - nature’s strategy is to get it out.
“Before discovery in 2007 of deuterium damaging mitochondria, there was a conference in the early 70s, where they looked at deuterium and said: "Well there's a term called the 'kinetic isotope effect'. The kinetic isotope effect explains how quickly something dissociates from something else. So in this case if you have a carbon hydrogen bond versus a carbon deuterium bond that carbon deuterium bond will dissociate nine times slower deuterium just slows things down. We know that but what else does it do well, because it's double the mass of hydrogen. If it gets into a position where something wants hydrogen, a molecule wants hydrogen and it's a deuterium it could distort the shape of that molecule, because it's just too heavy for that position and you see this in stereoisomers enzymes or stereoisomers. They have curality which is like you can see a picture of it. It's almost like if you look at an airplane: you know you have two wings and those wings are perfectly balanced and they weigh the same. Now imagine you made one wing slightly heavier that nothing looks different it's just slightly heavier. it's going to be a little harder to fly. You might crack. And this is what happens with enzymes and it's what happens with DNA because it just takes a position that's not meant for it. Now in the human genome there are three billion base pairs of DNA that means 10 million deuterium atoms in our genome. If you have too many deuteriums taking positions of hydrogen it will distort the shape and that shape is very important for its replication so you have errors in transcription with too much deuterium in your dna and this is what causes mutation”
“Even at very low levels. deuterium can slow down DNA replication processes or otherwise interfere with the repair of DNA damage by solar radiation” - very brilliant scientists in Eastern Europe found that the reduction of deuterium actually improves your not only resistance to stress, but actually radiation. Not only solar radiation, but all radiation: alpha, beta, gamma.
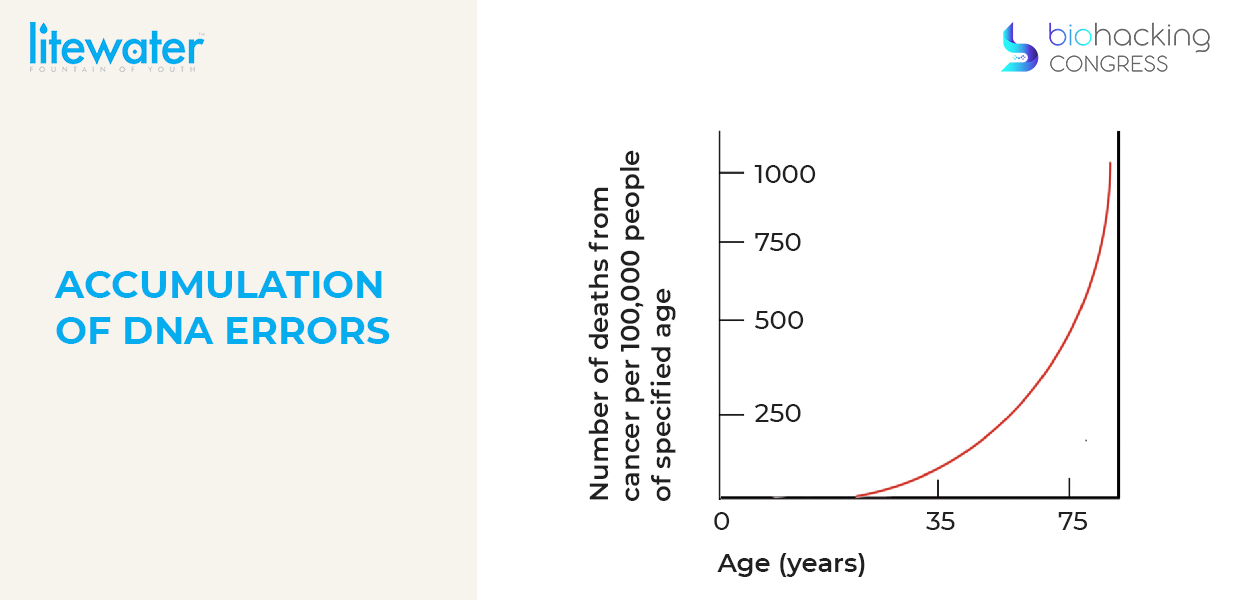
Another simple graph is just looking at aging. This defines the aging number of deaths from cancer per 100 000 people of specified age you could see as the accumulation of DNA errors go up so do all kinds of pathologies things just start breaking down.
“This is really profound because the studies show that to reduce the deuterium in our bodies you need half the amount of oxygen to perform the same amount of work. And it's probably the reason why in the Himalaya people can go to the summit without supplemental oxygen. Because their deuterium is depleted so they use the oxygen they have more efficiently, they need less oxygen to perform more to perform the same amount of work. So the climbers that go to the base camp mount Everest, where that glacier is in the upper 120 ppm range and they stay there they acclimate. They think they're acclimating to the elevation but they're really acclimating to the water. They drink this water and then they can go to the summit without supplemental oxygen too.
A strategy for longevity
It has been documented that practicing fasting regularly and consum naturally deuterium depleted glacial melt water are the healthiest and most long lived people on the planet.
Could deuterium depletion be the reason humpback whales get so big and live long? In Alaska they drink lots of glacial water in the summer and then fast for months in the winter burning fat for fuel as their deuterium depleted.
Camels live twice as long as horses, and can walk for a week in the hot desert without water. They follow a deuterium depletion strategy, making metabolic water from the stores in their humps.”
“Keto diet is a strategy for deteriorated depletion. Why? Because fats have less deuterium than carbohydrates and also for every 2.4 pounds of fat you produce one liter of deuterium depleted water. Because those fats are already depleted and this is why you should do this. Is why you should be more keto or keto adapted or focus more of your diet from fats. Because one ketone body will produce 104 molecules of atp whereas one glucose molecule will produce 34 molecules of ATP. That's if you want to know why you want to go keto.”
“The goal is to drink deuterium depleted water, practice a lifestyle that's more keto adapted so you can reduce the deuterium levels to where they're more manageable, so you can increase your lifespan and your health span. We test it, we have our own lab, it's deuteriumtest.com and we use a very complex piece of equipment, so you can test yourself, you can test your saliva and we do that. You can get a baseline and then you could later you can see if deuterium depletion is working for you. So after a month to three months you get to that range where you want to be and you stay there because it feels good so that is why deuterium depletion may be the most important health and medical discovery of our time.”
BiohackingCongress Team is very grateful to Victor Sagalovsky for joining the biohacking conference and giving an incredible lecture.
If you want to get Litewater with a discount, go to our website at the Top Biohacks section
Watch full Victor Sagalovsky's 40-minute lecture and 50+ lectures, panel discussions, and performances from renowned biohackers on biohacking your body with a BiohackingCongress year-long subscription. Get your access to a healthy future now!
Subscribe to watch more Videos with Top Biohackers. Stay tuned into the latest biohacking products for biohacking your body and biohacking for longevity.
Based on the lecture by Victor Sagalovsky
Special Access to
Exclusive TopBiohacks
and more



