The Science of Spermidine and its Role in Longevity
“My name is Don Moxley. I'm trained as an exercise physiologist. I've taught university for 25 in about the last 35 years but also worked in the industry. So I wind up spending a lot of time teaching in industry and that's why we're here today, we're gonna be talking about a molecule called Spermidine.
And so what we talk about
-learn what spermidine is;
-how Spermidine works in the body;
-understand the relationship between Spermidine and longevity.
Anton Van Leeuwenhoek was the first to explore the description of a crystalline form in semen. This is where it started. About 200 years later, a group of German scientists actually named it Spermidine. The research was continued by Dr. Madera, who was one of the Founders of Longevity labs, the company that I work with.”
What is Spermidine?
“Spermanite is called a polyamine. The three blue nodes are amino. So that's a nitrogen molecule with the accompanying hydrogen and with spermicide in, we have seven carbons in there. If we look at the science of all this, this gets a little bit overwhelming from a standpoint but it starts from an amino acid.
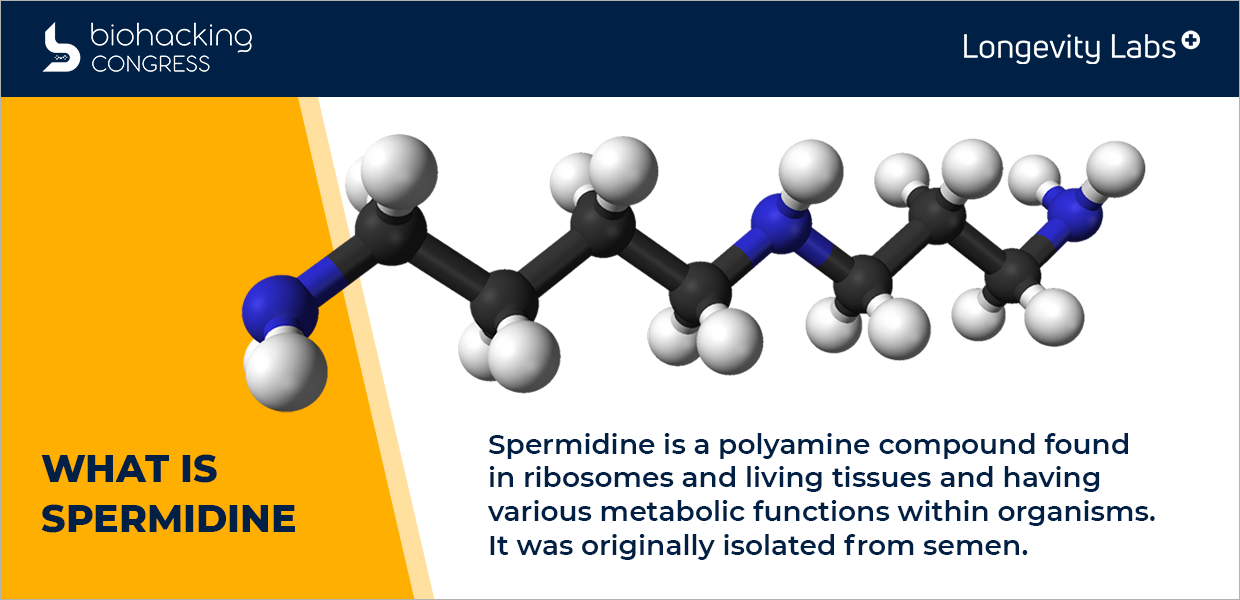
An amino acid is to aiming groups with a carboxy group is what makes it an acid. So you lose that, you lose the acid group. Putrid and it's simply it's the same molecule that just loses its acid. There are two amino groups. This is the first polyamine then through an enzyme that's called spermidine synthesis that we add another amino group. So this is where spermidine comes in its polyamorous. And there's the third polyamory, which has four amino groups. So that's kind of the chemistry, but understand that we have biosynthesis of this coming through an amino acid pathway. We have metabolism or digestion of it. So this is a very active molecule and we have transported it into it.
Regulation of the intracellular spermidine pool. Major routes and enzymes of mammalian polyamine metabolism. The cytosolic spermidine pool results from uptake, biosynthesis, catabolism, and transport. Spermidine is formed from its precursor putrescine or by degradation from spermine. Polyamine biosynthesis connects to arginine and NO metabolism via ornithine (as part of the urea cycle). Spermidine catabolism is mainly mediated through acetylation and subsequent oxidation reactions. Through the cofactors dcSAM (polyamine biosynthesis) and acetyl-CoA (polyamine catabolism), polyamine metabolism interrelates to protein/DNA methylation and protein acetylation, respectively, and thus indirectly influences epigenetic regulation of gene expression”
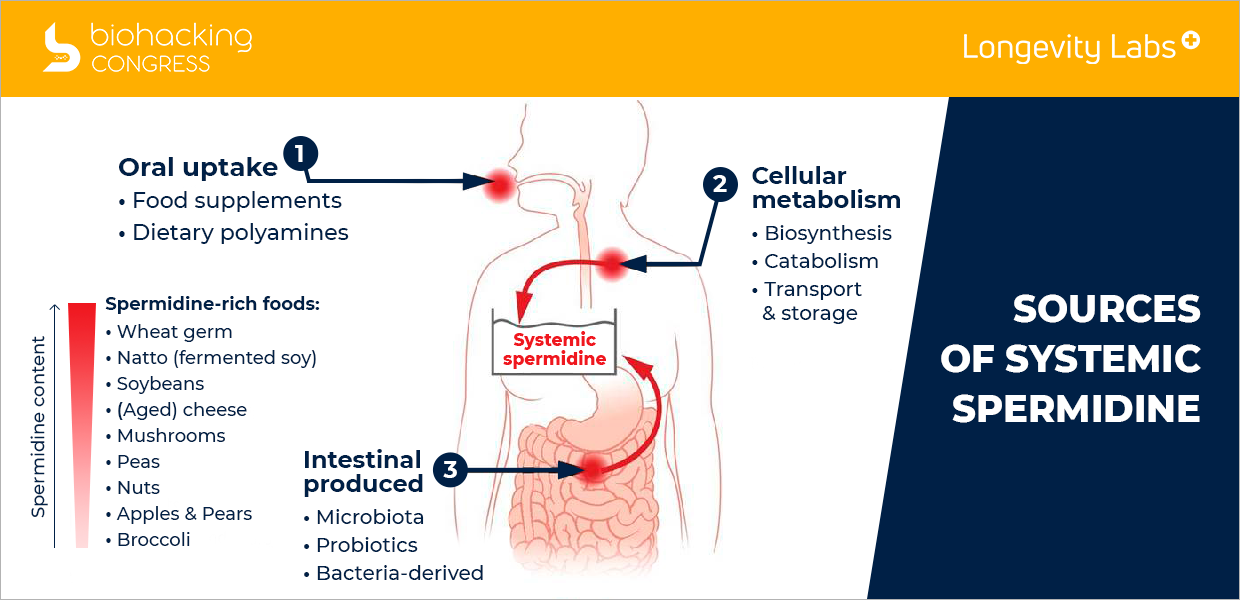
How do we get spermidine in our diet?
“Sources of systemic spermidine. Scheme depicting the sources crucial for spermidine bioavailability in the whole organism. In addition to cellular metabolism, spermidine is taken up orally from dietary sources or produced by commensal gut bacteria. Subsequently, spermidine can be resorbed by intestinal epithelial cells and is distributed through the systemic circulation.
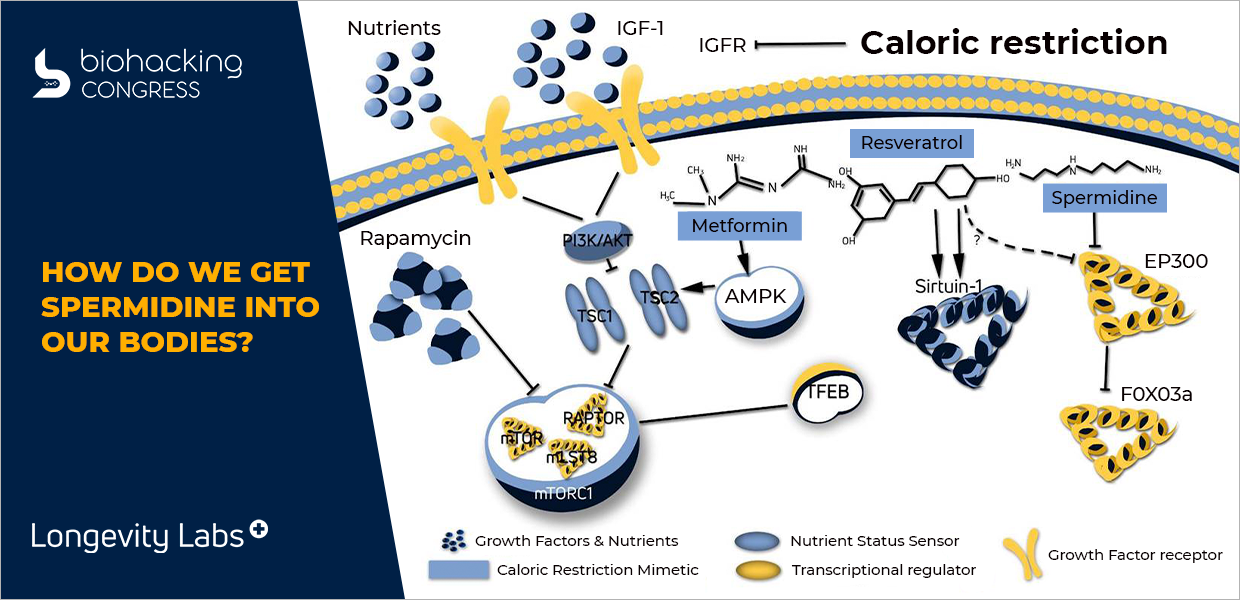
I want you to think of a topology. A lot of times we talk about topology as a destination. I want you to start to think about topology as the base state. It's the lowest state of the cell. If I take a body and remove all nutrients the response will be the production of a tautology. That is where fasting comes into play.
When I add nutrients back into the equation what I have here is I have the production of insulin and insulin-like growth hormone which comes down and eventually goes into what's called an M2 and says "Hey, make more proteins. We have nutrients onboard. Make more proteins."Now when I don't when I have calorie restriction and I don't have the production of insulin and insulin-like growth hormone." What we have is the cell says "Wait a minute, we don't have nutrients coming on board. We got to clean ourselves up". Well, that's what your body's doing with proteins. So proteins are made up of amino acids. When I have nutrients onboard and my M2 is making protein, it's going into the nucleus, it's grabbing some D. N. A.”
“Does that go into the ribosomes? Ribosomes say turn this nucleic acid pathway into a protein by bolting these amino acids together. Not all of those amino acids bolt together correctly. So there will be proteins that are created that are not functional. And if you don't clear those for the cell they become "long-lived" proteins. These are proteins that kind of work as we engage in a topology.
This is a set of genes that activate and we actually see those genes. If you've read lifespan, you'll see those genes actually move in your life from the reproductive phase of your life and the nucleus out into the longevity part of your life and the site is all it actually moves. So we know the body is smart enough to work between growth reproduction and longevity. And this is important. I like to think about our lives when I'm talking to people
We have genetic triggers. We have reactive oxygen species that are kicking in heat. Well, we're starting to take a look at the trigger. So any kind of stress to the cell. One of the things about the Covid virus. That's interesting. So we have a viral ontology trigger. So if you get a virus in the cell it triggers an autopsy process viral ontology also triggers an inflammatory response. It says to yourself "Hey, there's something going on here, let's get really protective". One of the challenges with COVID is COVID blocks the list is out of phase junction. So if you've got a cell that's not engaged in regular autophagy and you have a Covid infection. This is where you start to get to build up in the cell. So what you're getting is you're getting a trigger for immune response, you're getting these inflammatory triggers. And probably creating other viruses. I'm not quite sure. I'm not enough of a viral scientist. But this is the key place where Covid starts to play. If you have upregulated autophagy from other places, it'll finish the autopsy process. People who fast do better with covid than people who don't fast. People who have upgraded the immune response to better. So this is one of the values when we start to take it and this is kind of where it's happening in the cell.”
Cellular and molecular mechanisms of spermidine-mediated health protection.
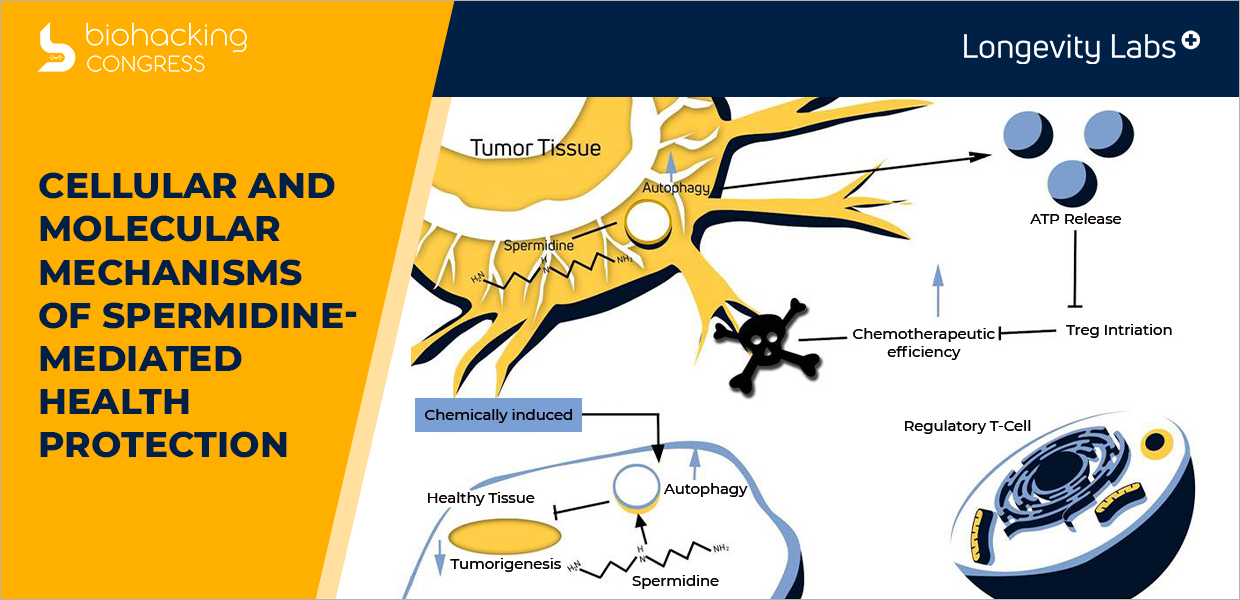
“Cellular and molecular mechanisms of spermidine-mediated health protection. Mechanistic models of the cellular and molecular effects elicited by spermidine through transcriptional, posttranslational (affecting protein acetylation and phosphorylation), as well as metabolic effects. Rapid autophagy induction by spermidine administration through inhibition of the acetyltransferase EP300, primarily resulting in autophagy-relevant cytosolic protein deacetylation. Sustained autophagic control by spermidine is mediated through induction of autophagy-relevant gene transcription. This involves the regulation of FOXO transcription factor as well as inhibition of histone acetyltransferases, resulting in epigenetic transcriptional reprogramming.
Spermidine may suppress tumorigenesis through induction of autophagy in healthy cells. In autophagy-competent tumor cells, spermidine favors the autophagy-dependent release of ATP, which in turn favors immunosurveillance.
Anti-inflammatory effects of spermidine are explained through its effects on macrophages, promoting M2 polarization and the suppression of NFκB-dependent proinflammatory cytokines. These inhibitory macrophages then suppress autoimmune-reactive T cells. At the same time, spermidine favors the formation of CD8+ memory T cells via induction of autophagy. The suppression of circulatory cytokines, such as TNFα, also contributes to cardiovascular protection, possibly via a concerted action with arginine-derived nitric oxide, which leads to vasodilation and promotes the cGMP/cGMP-dependent protein kinase (PKG)–dependent phosphorylation status of titin. Spermidine-enhanced autophagy and mitophagy also contribute to cardiomyocyte elasticity and mitochondrial functionality. The inhibitory effect of spermidine on autoimmune-reactive T cells further translates into the prevention of neurodegeneration by demyelination. Neuroprotection is also mediated via autophagy-dependent proteostasis of presynaptic active zones, assuring the maintenance of synaptic plasticity. Suppression of stem cell senescence by spermidine depends on autophagy (in satellite cells), whereas spermidine promotes mitochondrial function and the production of keratins in epithelial stem cells. These stemness-enhancing effects ensure muscle and hair follicle regeneration, respectively. Red arrows (up or down) indicate changes (increased or decreased, respectively) that are observed after spermidine supplementation.”
Spermidine-mediated health effects
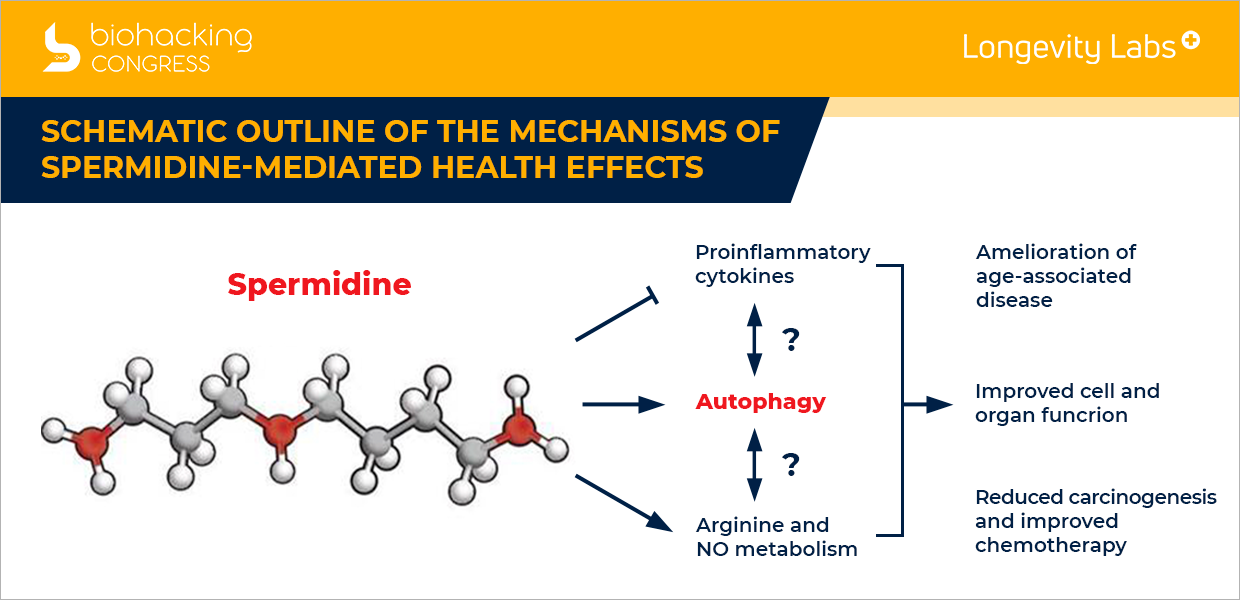
“Schematic outline of the mechanisms of spermidine-mediated health effects. The natural polyamine spermidine has prominent cardioprotective and neuroprotective effects, ameliorates aging-associated metabolic decline, and stimulates anticancer immunosurveillance in animal models. Autophagy is required for several of these health-promoting effects of spermidine. Spermidine also suppresses proinflammatory cytokines and improves the bioavailability of arginine required for NO biosynthesis. It remains an open question whether all these effects depend on the autophagy-stimulatory properties of spermidine.”
BiohackingCongress Team is very grateful to Don Moxley, Director of Applied Science and Brand Development, Longevity Labs INC. for joining the biohacking conference and giving an incredible lecture.
If you want to try SpermidineLIFE® by Longevity Labs with a discount, go to the Top Biohacks section
Watch Don Moxley’s 40-minute lecture and 50+ lectures, panel discussions, and performances from renowned biohackers on biohacking your body with a BiohackingCongress year-long subscription. Get your access to a healthy future now!
Subscribe to watch more Videos with Top Biohackers. Stay tuned into the latest biohacking products for biohacking your health and biohacking for longevity.
Based on the lecture by Don Moxley
Special Access to
Exclusive TopBiohacks
and more


